Cruxi

510k submission services
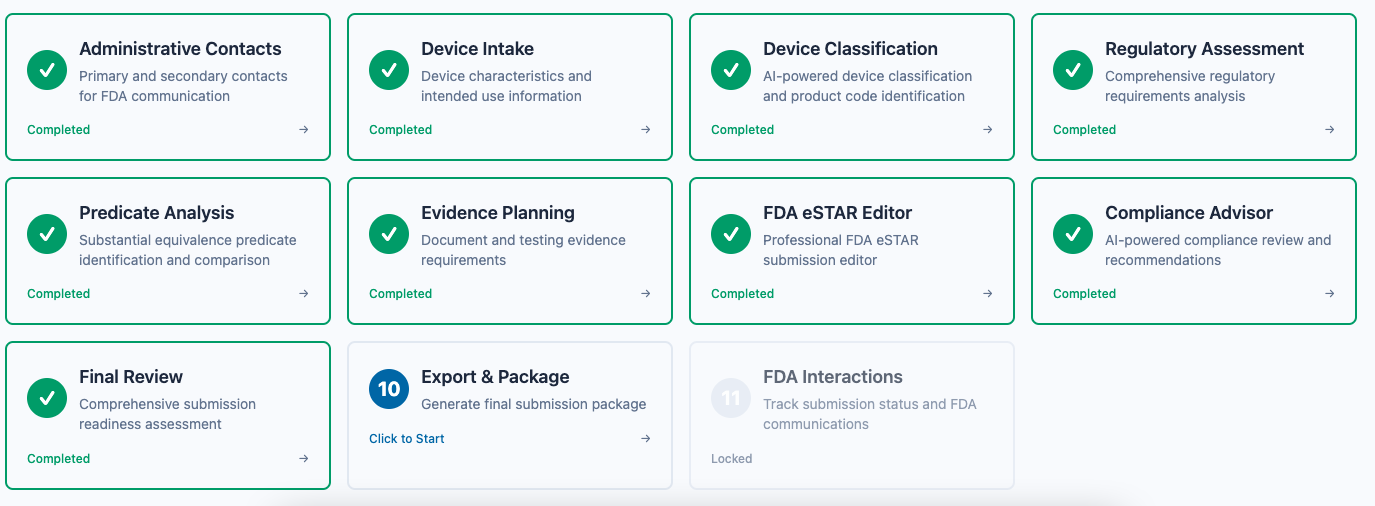
Cruxi – Automated 510(k) submission platform for medtech regulatory processes
Summary: Cruxi is an AI-powered platform that automates FDA 510(k) submissions by classifying devices, analyzing predicates, assessing regulations, and drafting eSTAR-ready documents. It streamlines complex regulatory workflows to help medtech teams accelerate FDA clearance and improve submission quality.
What it does
Cruxi uses a chain of AI agents to classify medical devices, identify relevant predicates and guidance, outline testing and documentation requirements, and generate draft eSTAR sections for review by regulatory teams.
Who it's for
It is designed for medtech founders, regulatory affairs and quality assurance teams, and consultants working with FDA 510(k) submissions and related regulatory documentation.
Why it matters
Cruxi addresses the complexity and high cost of 510(k) submissions by providing structured, actionable regulatory insights and draft content, reducing delays and reliance on expensive outsourcing.
